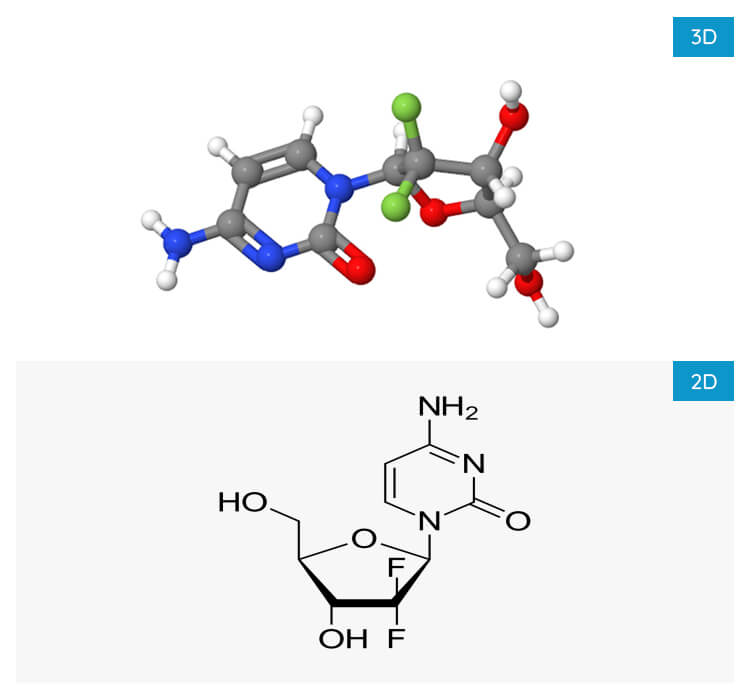
Gemcitabine Hcl
Overview
Gemcitabine,is a chemotherapy medication used to treat cancers. It is used to treat testicular cancer, breast cancer, ovarian cancer, non-small cell lung cancer, pancreatic cancer, and bladder cancer. It is administered by intravenous infusion. It acts against neoplastic growth, and it inhibits the replication of Orthohepevirus A, the causative agent of Hepatitis E, through upregulation of interferon signaling.
Key Features
- Molecular FormulaC9H11F2N3O4
- Therapeutic Category Nucleoside Metabolic Inhibitor
- API Technology High Potent
- Dose Form Injectable
Use In
-
Breast cancer
It is used as a second-line treatment in combination with paclitaxel for breast cancer that is metastatic or cannot be surgically removed.
-
Ovarian Cancer
It is used as a second-line treatment in combination with carboplatin for ovarian cancer.
-
Pancreatic Cancer
It is used as a first-line treatment alone for pancreatic cancer.
-
Bladder Cancer
It is used in combination with cisplatin for advanced or metastatic bladder cancer.
-
Lung Cancer
It is used in combination with cisplatin for advanced or metastatic non-small cell lung cancer.
| Open Part | Close Part | IP | BP | USP | EP |
|---|---|---|---|---|---|
| Available | Available |  |
 |
 |
 |